Table of Contents
Diesel Cycle
Diesel Cycle is a thermodynamic cycle that models how a diesel engine operates. It’s a type of internal combustion engine that uses compression burning, where fuel is injected into the cylinder and burning by high temperature and pressure of the compressed air.
In simple words: The diesel engine works by compressing air, injecting fuel and converting thermal energy into mechanical work.
Working Principle of Diesel Cycle
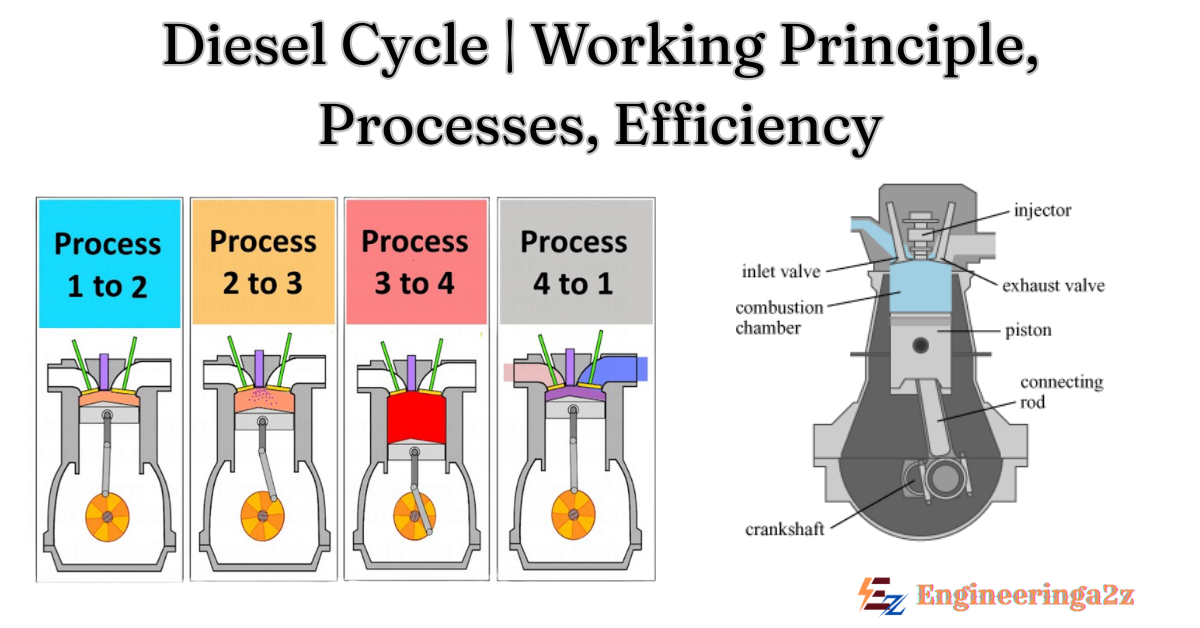
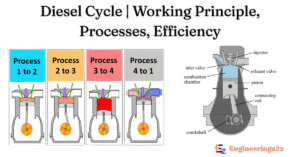
The diesel cycle consists of four main processes :
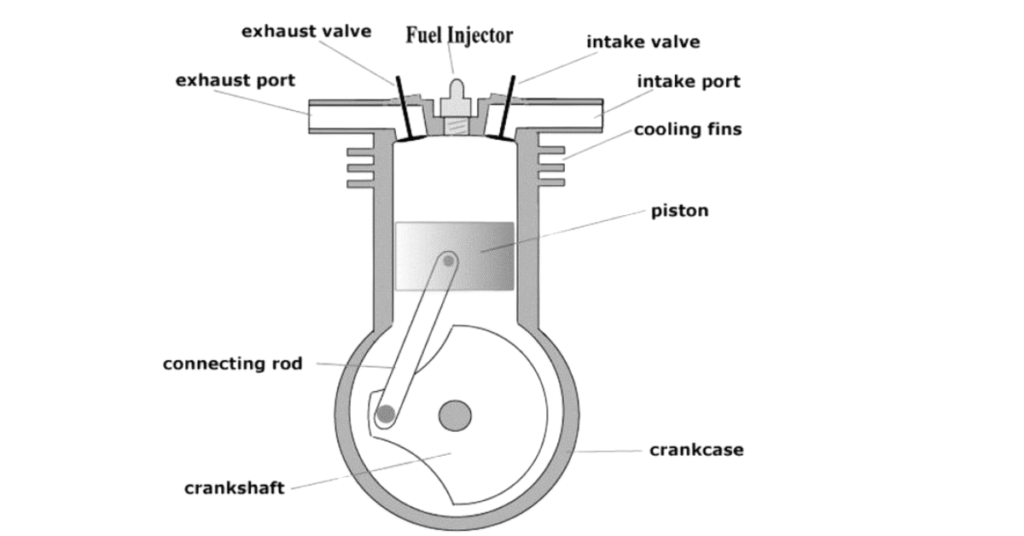
- Intake (0-1): The piston moves down, drawing fresh air into the cylinder through the open intake valve. This happens at constant pressure.
- Compression (1-2): The intake valve closes and the piston moves up, compressing the air. This process is adiabatic, meaning no heat is exchanged with the surroundings. The temperature and pressure of the air increase significantly.
- Combustion (2-3): Near the top of the compression stroke, fuel is injected into the hot, compressed air. The high temp. causes the fuel to ignite spontaneously. Combustion occurs at approximately constant pressure as the piston is pushed down. This is the heat addition phase.
- Expansion (3-4): The hot, high-pressure gases expand, pushing the piston down and doing work. This is another adiabatic process.
- Exhaust (4-1): The exhaust valve opens and the piston moves up, pushing the exhaust gases out of the cylinder. This occurs at approximately constant volume.
Thermodynamic Process of Diesel Cycle
An ideal diesel cycle have four thermodynamic processes: two isentropic (reversible adiabatic) processes with one constant pressure combustion process and one constant volume heat rejection process.
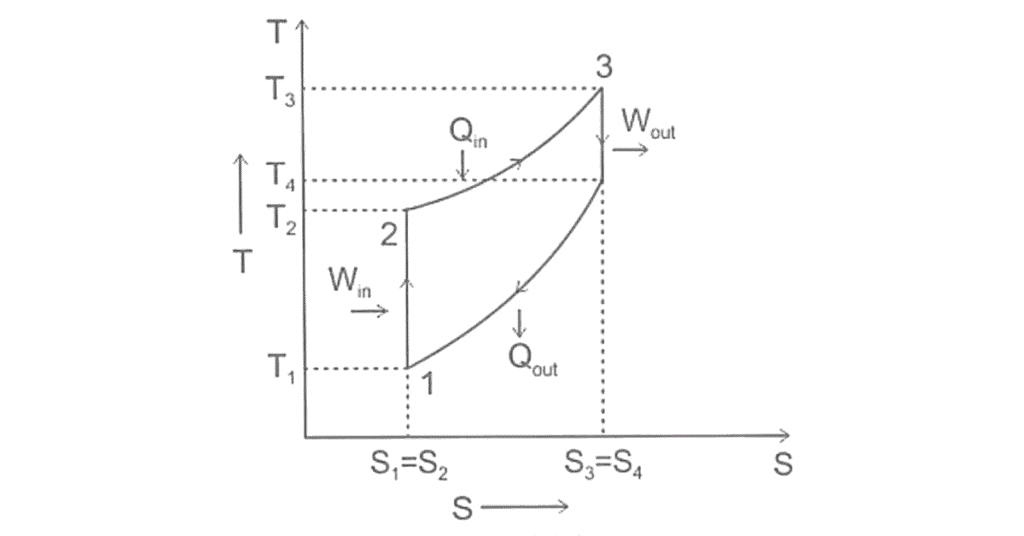
Graphical diagram is based on thermodynamic processes Pressure-Volume (PV) Diagram and Temperature-Entropy (TS) Diagram.
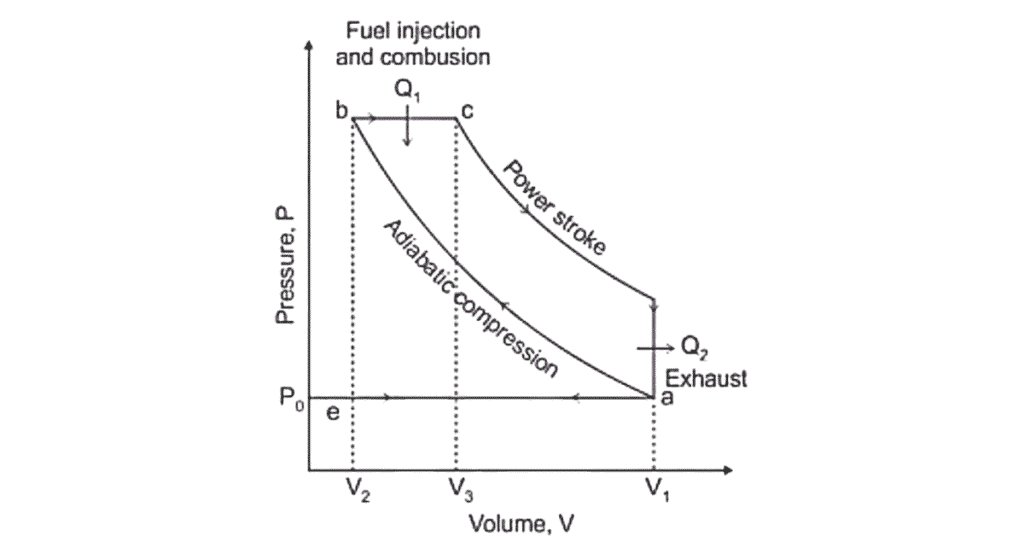
1. Isentropic Compression
In isentropic compression, air is compressed to high pressure and temperature. In this process, both pressure and temp. are high due to compression. Entropy remains unaffected.
During isentropic compression, ΔQ = 0.
For an ideal gas, ΔH = V. ΔP ⟹ W = H2 − H1 ⟹ H2 − H1 = Cp
Here, Cp = Constant Pressure & H = Enthalpy.
2. Constant Pressure Combustion
In constant pressure combustion fuel is injected, ignited and burns at constant pressure, increasing volume. When pressure is constant their volume is increased
In this process, V.dp = 0 ⟹ dH = dQ → Q = H2 − H1
According to Ideal gas law pressure inversely proportional to volume and directly proportional to quantity and temperature. Thus, the constant pressure combustion is stated as,
V/T = Constant.
3. Isentropic Expansion
In isentropic expansion , the hot gases expand, producing work. In this process when hot gases are increasing then work is produced. The entropy remains constant. It is also known as isochoric process.
Now, V4/V3 is the isentropic expansion ratio.
4. Constant Volume Heat Rejection
In constant volume heat rejection, heat is rejected, returning to the initial state. In this process when volume is constant their heat is rejected after rejection its reached on its initial state.
For an isochoric process, P. dV = 0 ⟹ dU = dQ ⟹ dU = 0 = Q – W ⟹ W = Q.
Efficiency of Diesel Cycle
The diesel engine works on the diesel cycle is known as heat engine (heat energy is converted to work).
For any heat engine, the thermal efficiency ηth is equal to the ratio of work done ‘W’ by Qadd. Mathematically,
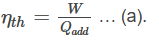
According to the first law of thermodynamics, energy cannot be completely converted into work. Therefore the heat input ‘Qadd’ equal to the work done ‘W’ and an amount of heat released as waste heat ‘Qout’
So equation (a) can be written as
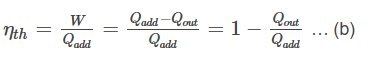
During an isochoric process, work done on or by the system is zero. By the first law of thermodynamics, ΔU = ΔQ. Means heat addition and rejection equations are equal,
Qadd = m.Cp (T3 – T2 ) & Qout = m.Cv (T4−T1)
Substituting these in equation (b),
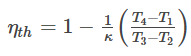
This is the diesel cycle efficiency formula.
Here, k = Heat capacity ratio

Let us discuss some ratios that we just came across in the diesel cycle derivation.
1. Compression Ratio
Compression ratio higher than in a gasoline engine, usually between 18:1 and 22:1. This higher compression ratio has many benefits like ignition without spark plug, efficient combustion, better fuel efficiency.
2. Fuel Cut-off Ratio
The ratio of the cylinder volume at the end of fuel injection to the cylinder volume at the start of fuel injection. Fuel cut-off ratio affects the engine performance. It can also impact the engine emissions.
3. Heat Capacity Ratio
The heat capacity ratio, also known as the adiabatic index or isentropic expansion factor, is the ratio of the specific heat capacity at constant pressure (Cp) to the specific heat capacity at constant volume (Cv).
Applications of Diesel Engine
- Trucks and Buses: Due to their high torque and fuel efficiency.
- Trains: For powering locomotives.
- Ships: For propulsion.
- Construction Equipment: Such as bulldozers and excavators.
- Generators: For producing electricity.
- Agricultural Machinery: Such as tractors and combine harvesters.
Frequently Asked Questions (FAQs)
-
What is diesel cycle also called?
Diesel Cycle is a thermodynamic cycle describing the operation of a compression ignition engine, where fuel is ignited by the high temp. of compressed air.
-
What are the four processes of the diesel cycle?
There are four stages of the diesel cycle:
1. Isentropic Compression
2. Constant Pressure Combustion
3. Isentropic Expansion
4. Constant Volume Heat Rejection -
Why is the diesel cycle more efficient than the otto cycle?
Because of its higher compression ratio, which results in higher thermal efficiency and better fuel utilization.
Related Posts
- Electrical Power Notes PDF (Handwritten) | Power Plants, Generation Economics & Transmission
- MTech Power System Previous Year Question Papers PDF
- Thermodynamics Notes PDF (Handwritten) | Simple & Exam Oriented
- Non-Conventional Sources of Energy Notes PDF (Handwritten)
- Ground Fault Indicators: How they Work, Where they Fit and What to Look for
- Diesel Cycle | Working Principle, Thermodynamic Processes & Efficiency











Leave a Reply