
Table of Contents
Fuel Cell
Fuel Cell is a cell that converts the chemical energy of fuel into electrical energy directly without any combustion.
OR
It is a energy storage device in which chemical energy of a fuel is converted into electrical energy by an electrochemical process.
Types of Fuel Cell
There are following types of fuel cells :
- Hydrogen oxygen fuel cell
- Hydrazine fuel cell
- Hydrocarbon fuel cell
- Direct Methanol fuel cell
Operation Principle of Hydrogen Oxygen Fuel Cell
Fuel cells can be explained by using hydrogen-oxygen fuel cell. It consists of
- Two porous metal electrodes
- An electrolyte
Platinum metal is used as electrodes for military and space applications. Sometime porous nickel electrodes and porous carbon electrodes are also used as fuel cell electrodes.
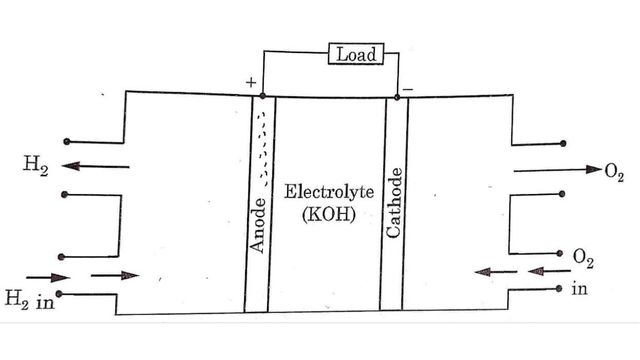
In hydrogen oxygen fuel cell, hydrogen is the active material at the anode i.e. (+ve electrode) and oxygen is active material at the cathode i.e. (-ve electrode). Between the electrodes a layer of electrolyte is filled. Electrodes are made of porous material.
Hydrogen gas is supplied to the anode electrode whereas oxygen gas is supplied to the cathode electrode. Then electro chemical reactions occurs between electrodes and electrolyte as follows:
At anode
H2 →2H+ + 2e–
H2 molecules break into H+ ions at anode. These H+ ions combine with hydroxyl (OH-) ions to form water and produce electrons at the anode.
2H+ + OH– → H₂O+ e–
These electrons travel to the cathode through external circuit while H₂O formed migrates toward cathode.
At cathode
Oxygen atoms combine with four electrons and water to form Hydroxyl (OH-) ions.
O2 + 2H2O + 4e → 4OH–
These OH- ions move towards the anode as shown in Fig
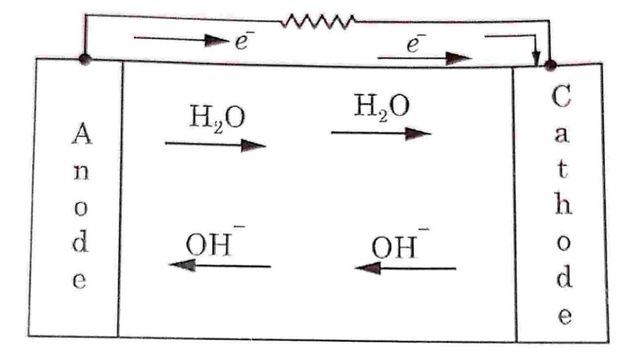
Hence e.m.f. will be developed between anode and cathode electrodes. When external load is applied across the electrodes, electrons will move toward cathode through external load.
Hence current will flow in a direction opposite to the direction of movement of electrons.
Direct Methanol Fuel Cell
Direct methanol fuel cells or DMFCs are the subcategory of proton-exchange fuel cell in which methanol is used as a fuel.
Methanol (CH3OH) liquid is used at temperature from -97 °C to 64.7°C under atmospheric pressure. Methanol is toxic and flammable.
Construction and Working of Direct Methanol Fuel Cell
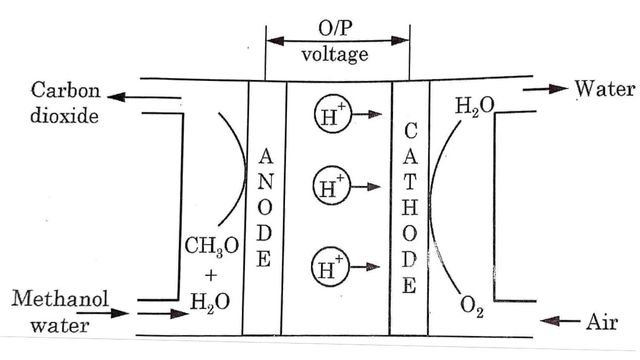
Direct methanol fuel cell is a cell that runs directly on methanol without having to first convert into Hydrogen gas. It consists of two electrode separated by a proton exchange membrane (PEM). At anode methanol and water enter and lose the protons until carbon dioxide is formed.
At anode
CH3OH + H2O → 6H+ + CO2 + 6e–
Water is consumed at the anode in the reaction and Hydrogen gas is produced. Similarly oxygen enters at the cathode electrode and forms water.
At cathode
3/2 O2 + 6H+ + 6e– → 3H2O
Overall reaction
CH3OH + 3/2 O2 → 2H2O + CO2
It is easy to transport but it’s efficiency is low as compared to other fuel cells. So, it is used for particular applications only.
Advantages of Fuel Cell
- It has high conversion efficiency i.e. 50 to 60%.
- It is simple and safe.
- Fuel cell can be installed near the load point.
- It has no moving parts.
- It is pollution free method to generate electricity.
- It is compact and light in weight.
- It takes little time to get into operation.
Disadvantages of Fuel Cell
- It has high initial cost.
- Hydrogen is hard to store and transport safely.
- Fuel cells don’t last very long and can lose performance over time.
- There are very few hydrogen filling stations available.
- Most hydrogen is made from fossil fuels, which is not environment-friendly.
- Fuel cells can be affected by temperature and fuel impurities.
- The system is complex and needs skilled people for repair and maintenance.
Applications of Fuel Cell
Various applications of fuel cell given below:
- Domestic use.
- Power stations.
- Automotive vehicles.
- Special applications.
Related Posts
- Difference Between HVDC and HVAC
- Different Types of Faults in Overhead Transmission Lines
- Circuit Breaker | Types of Circuit Breaker
- Tariff: Definition, Types, Objectives & Characteristics of Tariff
- AC and DC System : What is AC and DC Transmission System
- Buchholz Relay | Construction and Working Principle














Leave a Reply